Sustainability
Society
Bonds with Customers
Provide Value for Society, Using High Quality Pharmaceutical Products and Services Backed by Unique Technology
Since the development of SalonpasⓇ, the Hisamitsu Pharmaceutical Group has continued to develop and improve our products by promptly responding to customer needs. At the same time, Hisamitsu has refined the TDDS technology (including the Transdermal Patch Technology), to create a variety of products such as Rx drugs, OTC drugs, and skincare products. Going forward, we will not only spread the “Patch Treatment Culture” of our transdermal patches but will also promote the “TE-A-TE” culture worldwide and conduct research and development on a global platform to deliver products that meet the needs of our customers in a timely manner. Constantly thinking about “Delivering a Better QOL to the World” with products that embody the spirit of “TE-A-TE,” we shall provide more effective and safer products and services to the world.
Value to Be Presented to Society
Since our establishment, we have been engaged in product development to satisfy customers’ needs in accordance with the basic policy of “The Customer-first Principle,” with our main focus on transdermal patches through which pharmaceuticals can be administered on a stable basis and which can be patched with comfort. In addition, we promptly reflect customer needs into our products and quickly respond to changes in the social environment, such as the aging population. To do so, we conduct product sampling to let customers and medical workers directly know about the excellence of our products, and also ensure cooperation between the actors in our value chain. While continuing to create value that will contribute to improving the environment and society, we will achieve global application of our technology developed in Japan so that we can present the world with new value that will contribute to citizens’ healthy and rich lives.
R&D
Based on the fundamental technology platform for TDDS that we have developed over many years, the R&D Division is working to develop TDDS formulations of drugs that are difficult to absorb through the skin. We are engaged in the research, development, and improvement of drugs that are clearly differentiated from existing and competing products by maximizing the benefits of TDDS formulations, such as stable efficacy and reduced adverse drug reactions resulting from sustained drug concentrations in the blood.
Additionally, in order to launch new pharmaceutical products in the global market as quickly as possible, we are striving to consolidate our R&D efforts made at our domestic R&D sites and our overseas subsidiary (Noven Pharmaceuticals, Inc.) and shorten the development pipeline period.
Operation of the New Research Lab, the SAGA Global Research Center, started
In February 2024, the SAGA Global Research Center was completed and started full-scale operation. The research functions, which had been located in Tsukuba City, Ibaraki Prefecture, and Tosu City, Saga Prefecture, in Japan before, were consolidated into the Center, aiming for improvement in the speed of development through collaboration between researchers and the maximization of the R&D function. In addition, the Center takes up the enhancement of collaboration with the production departments of the Tosu Factory, which is the mother factory of the Hisamitsu Group located near the Center as one of its important missions.
- [Overview]
- ・Consolidated our domestic research sites into a single location to speed-up research and development
- ・Set up a Common Laboratory to promote open innovation
- ・Acquired the ZEB Ready certificate*
*Reduced the building energy consumption by 53%
| Building area | 5,660㎡ | Start of construction | September 28, 2022 |
|---|---|---|---|
| Total floor area | 23,290㎡ | Completion Date | February 29, 2024 |



Development of a New R&D Organization
The main mission of R&D Division is to bring new products into the market and to develop existing products (additional indication, product improvement). In order to achieve the Seventh Medium-term Management Policy and solidify the future potential of Hisamitsu Group, we are restructuring the former R&D organization into a Group R&D organization centered on SAGA Global Research Center.
For the R&D organization, we reorganized the former research departments into five units. With this reorganization, we will promote R&D more efficiently, ranging from basic research to development and tests, including support for various modalities.
For global expansion of the Group, we will seek to promote flexible development in line with the trend and legal restrictions of each country or market, mainly led by the SAGA Global Research Center.
In addition, we have also established a structure for managing intellectual properties strategically by proceeding with collaboration with parties concerned at Shonan Health Innovation Park and promotion of open innovation.
5 units for Enhancing R&D
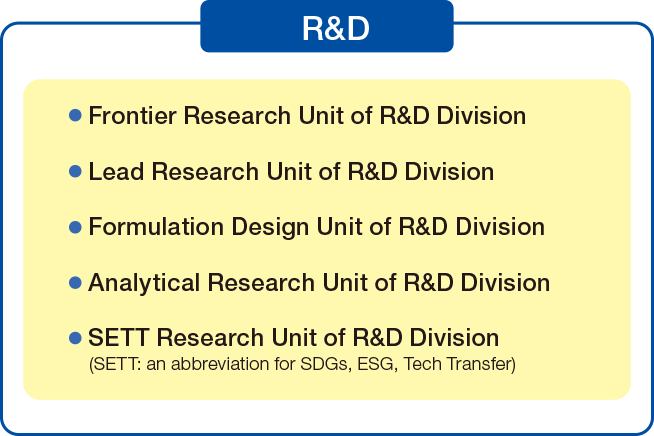
Drug Development
Hisamitsu Pharmaceutical Group is engaged in research and development of TDDS formulations (transdermal patches, gel, spray, and other topical formulations) to meet the fundamental needs of people around the world for “health, safety, and comfort” in their lives. By using TDDS formulations of drugs, we can respond to a wide range of medical needs regardless of the medical department, such as “maintaining appropriate drug blood levels for extended period,” “enabling drug administration to patients who have difficulty swallowing, such as children and the elderly,” and “allowing easy interruption of administration by removing or washing away the drug if adverse reactions develop.” In research and development, we are striving to develop highly useful TDDS formulations through exhaustive search for candidate drugs and active promotion of alliances. Furthermore, drugs are developed to meet the needs of healthcare community through the development of TDDS formulations that make full use of new fundamental technologies, such as microneedles for TDDS of a wider range of drugs, including polymeric drugs and vaccines.
“Promoting ‘TE-A-TE’ Culture Worldwide” with TDDS Technology
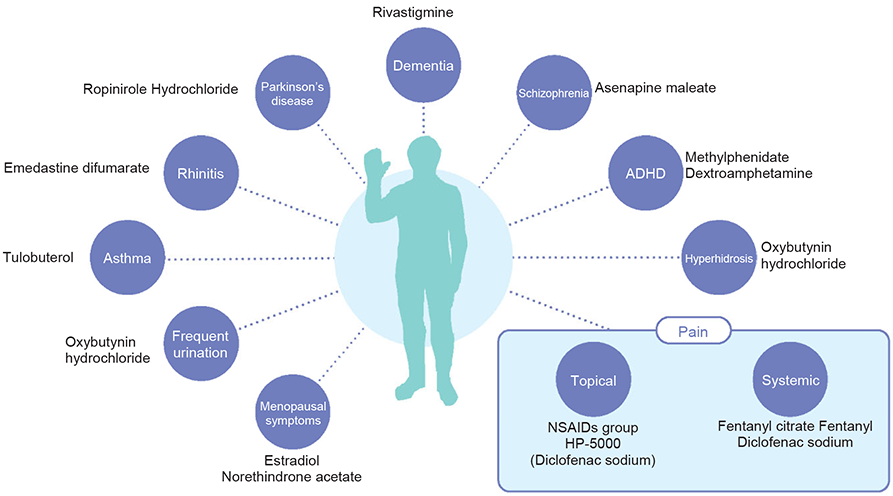
A microneedle is among the new technologies of TDDS. It is a new drug delivery system with an immediate effect and less invasiveness at the time of administration, which has not ever been achieved in the conventional dosage form. In the early phase II trial of the microneedle-type tranquilizer HP-6050, a placebo-controlled double-blind trial was conducted on patients with delirium, vibrational excitation, or irritability. As a result, its effectiveness was verified, and no side effect was observed that could cause safety issues in the development. On the other hand, making use of the pleasant stimulation sensation of the microneedles on the skin, we are also proceeding with the development of microneedles for cosmetic use. These developments are being prepared for commercialization with a view to partnership with other companies.We have provided products for various diseases, including the treatment drug for primary palmar hyperhidrosis APOHIDEⓇ Lotion, the transdermal treatment drug for overactive bladder NEOXYⓇ Tapes, and the transdermal treatment drug for allergic rhinitis ALLESAGAⓇ Tapes.At the same time, we are considering the development of various products and services that embody the spirit of “TE-A-TE” and have begun to take on the challenge of entering new areas such as health foods and therapeutic apps.We are conducting R&D activities based on the needs of healthcare communities, mainly focusing on the development of transdermal patches.For Rx drugs in the United States, we have proceeded with the development of a transdermal analgesics and anti-inflammatory drug HP-5000 (the generic name: Diclofenac sodium) for the indications of osteoarthritis of the knee; however, in order to accelerate the overseas expansion of ZICTHORUⓇ Tapes, we will newly promote the clinical development of HP-3150 (the generic name: diclofenac sodium) in the United States in place of the said development as an analgesic to meet the indications of chronic back pain. XELSTRYM™ (development code: d-ATS, the generic name: d-amphetamine), a transdermal treatment drug for attention-deficit hyperactivity disorder, newly received approval on March 22, 2022, and began the sales in the United States on June 5, 2023.
R&D Pipeline
Click here for information on our R&D pipeline.
Expansion of Target Diseases for Hisamitsu TDDS
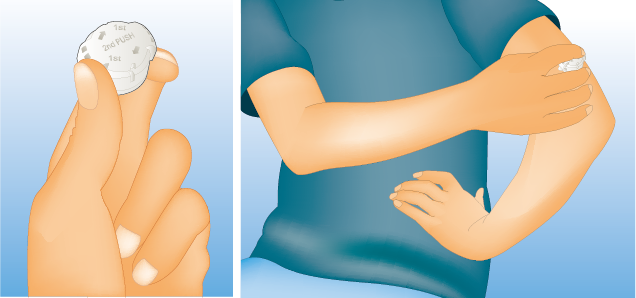
Microneedle Technology
HalDiscⓇ Technology
(Pinhold-shaped microneedle technology)
Realization of macromolecular drugs that are treated as injectable drugs such as vaccines, offering easy self-medication by anyone
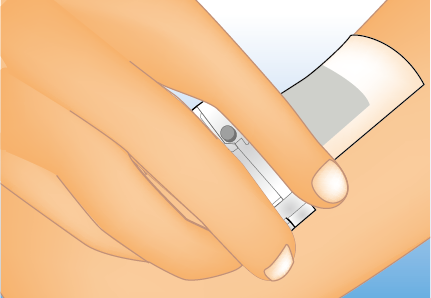
SheetifyⓇ Technology
(Sheet-type microneedle technology)
Hisamitsu's proprietary sheet-type device for the realization of high-dose transdermal administration
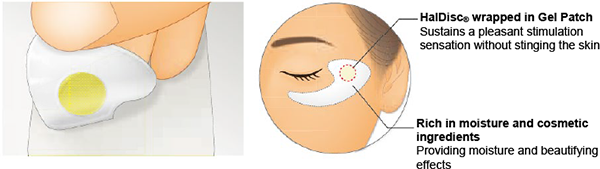
HalDisc beauteⓇ
(Cosmetic Microneedle Technology)
Hybrid technology for cosmetic use that combines transdermal absorption technology and microspike substrates to create a pleasant stimulation sensation
Drug Improvement
In the improvement of drugs, we have continuously worked to choose the size and shape fit for the purpose and reduce the frequency of use of drug products in order to improve QOL of our customers, mainly based on customer opinions (needs), the latest manufacturing technologies, and our research and development outcomes.
Until now, the focus has been on the perspectives of “manufacturers” and “users”, but it is important to proactively consider new perspectives as the Hisamitsu Pharmaceutical Group works to promote ESG and the SDGs. We will continue to work actively on the improvement of our products into ECO ones with the purpose of reducing CO₂ emissions and impact to the environmental circulation. In addition, the “standardization of product specifications” is being promoted as one of our measures to reduce environmental impact through a joint project among R&D, Production, and Sales divisions. Furthermore, from the perspective of open innovation, collaboration is promoted with suppliers, utilizing a broad range of technology information.
To ensure
Consideration for the Packaging of Rx Drugs
We have striven to develop eco-friendly and proper packaging also for Rx drugs. In 2023, improvement in MOHRUSⓇ Tapes 20mg and MOHRUSⓇ Tapes L 40mg was recognized with the Optimum Packaging Award of Japan packaging Contest hosted by Japan Packaging Institute and the Packing Technology Award of the Kinoshita Award.
The packaging awarded this time use 80% recycled PET for the packing pouches while ensuring the same quality as the traditional pouches, which is for the first time in Rx drugs. The amount of plastic usage decreased by 15.2% by reducing the thickness of the packing pouches through changing the composition of materials. The use of this PET is expected to have an effect of reducing CO₂ by 24% compared to PET used for the conventional products. In addition, we have changed white ink used for the packing pouches to 10% biomass one. We take environmental issues and others into consideration from various perspectives.
The Kinoshita Award: It is an award program established to commemorate the achievements that Mr. Matasaburo Kinoshita, the Second Caging Institute, accomplished in the packaging industry for many years.
Japan Packaging Contest:The best works in the year (Good Packaging Award) are selected from various functional areas of materials, plan, technology, appropriateness, eco-friendliness, design, packaging for transportation, logistics, sales promotion, and ideas.


Ethical Drug Development
Ethical and Scientific Clinical Studies
To ensure human rights and safety of patients participating in clinical studies, clinical trial protocols are prepared in compliance with the Pharmaceutical Affairs Law and the GCP*1 while obtaining informed consent from the patients. Additionally, the Internal Institutional Review Board is established to assess the ethical and scientific validity of studies with outside medical experts as members. When conducting clinical studies outside Japan, we observe the ICH-GCP*2 and comply with the regulatory requirements and guidelines of each country.
*1 GCP:Good Clinical Practice Standards for practice of clinical studies of drugs.
*2 ICH-GCP:International GCP Guidelines on the practice of clinical trials and clinical studies agreed at the Japan, the U.S. and EU ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use).
Ethical Accommodation for Animal Welfare
Hisamitsu Pharmaceutical has formulated the Animal Testing Guiding Principle in line with the Act on Welfare and Management of Animals to ensure that respect for animal welfare is completely shared and that animal testing is performed properly with accommodation for animal welfare. For animal testing to inspect drug efficacy and safety, we have a system requiring all such testing to be reviewed by the Animal Testing Committee, and research is conducted under the 3R Principles: Replacement, Reduction, and Refinement. In addition to conducting regular self-inspection, our animal testing operations underwent third-party review and are accredited as an animal testing facility. For the SAGA Global Research Center, we also plan to gain accreditation as an animal testing facility in the future.
Procurement
At our procurement departments, not only do we strive to maintain stable procurement of quality raw materials, reduce costs, and ensure that delivery deadlines are strictly met (and cope with natural disasters), but we also improve our supply chain, collect information from suppliers, and share such information with other in-house departments. From the phases of product planning and research, our procurement departments engage in discussions on raw materials in cooperation with related departments in order to establish a stable supply structure. At the same time, we strive to promptly reflect customer needs in our product development.
Based on our global strategies, we select appropriate raw materials so that people in many countries can use our products without worries, including worries about human rights risks in our supply chain and conformity with halal standards. We also procure items in light of their environmental friendliness, such as their contribution to reducing resource use, reducing waste generation, and increasing the use of recovered paper.
In December 2021, in response to the growing importance of sustainable procurement, the Company established the Sustainability Procurement Basic Policy and the Sustainability Procurement Standards for Suppliers, which suppliers are required to understand and comply with, as the Sustainability Procurement Guidelines.
Declaration of Partnership Building

This time, we announced the “Declaration of Partnership Building” on July 26th, 2023, in agreement with the aims of the “Council for the Building Partnerships to Lead the Future”, which is promoted by the Cabinet Office and The Small and Medium Enterprise Agency. We aim to build new partnerships by promoting cooperation and mutual prosperity with our suppliers in the supply chain and with businesses that create value.
Production
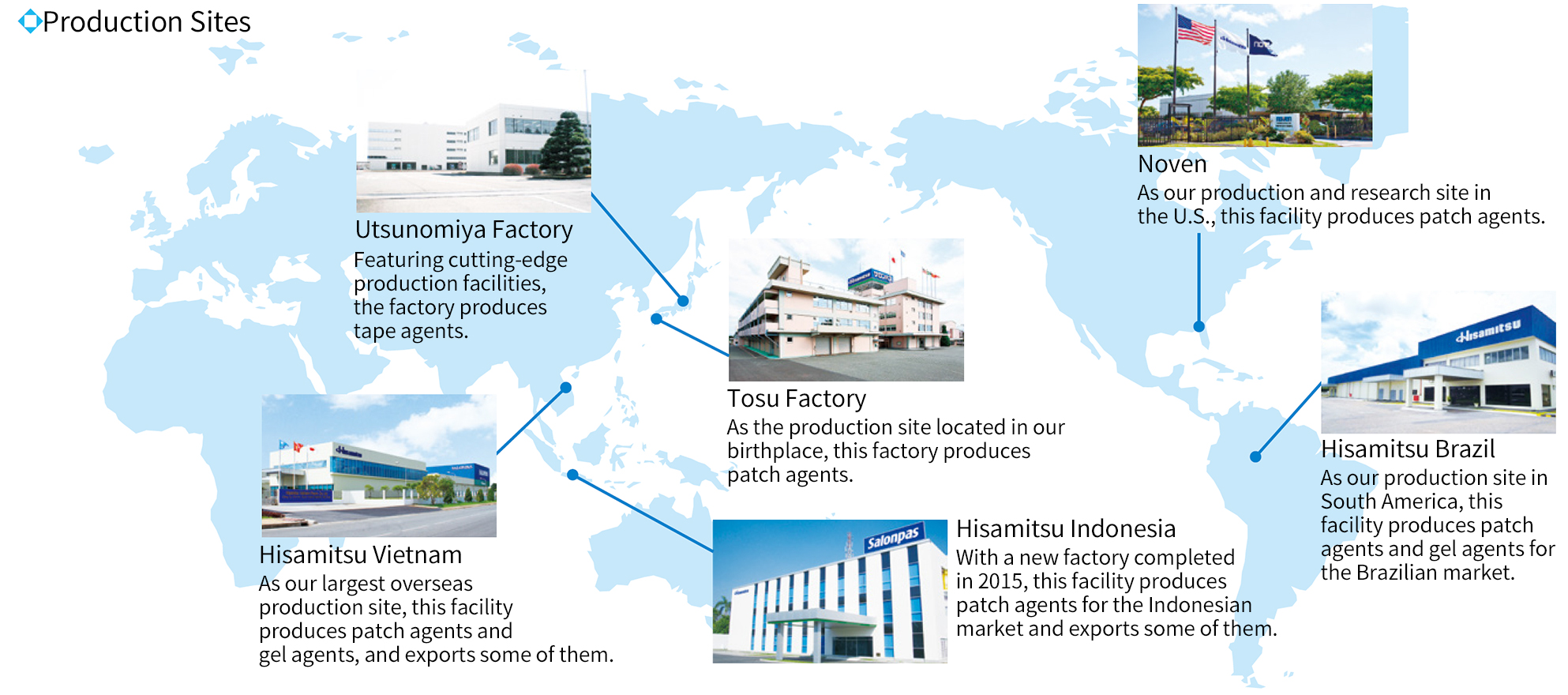
While ensuring cooperation with manufacturing contractors, our domestic production is centered on the Tosu Factory (Saga Prefecture) and the Utsunomiya Factory (Tochigi Prefecture), both of which are in compliance with the GMP*, so as to maintain a stable supply of products. In addition to ensuring a stable supply, we are also working to improve productivity. We are raising the efficiency of the entire production process not only by carrying out KAIZEN (improvement) activities on continual basis, but by reviewing the production system through elimination of production lines, improving individual processes, and increasing operating rates.
Moreover, we have introduced an IoT-based production system, thereby promoting KAIZEN activities using big data accumulated in the production system, and we will further improve productivity.
We globally sell pharmaceutical products, many of which are directly exported from Japan. To establish an even more stable supply system, however, we also have production sites in the U.S., Brazil, Vietnam, and Indonesia. While ensuring cooperation between our domestic and overseas production sites, we are localizing production and expanding our production capacity so that we can reinforce our global production system.
*GMP:Good Manufacturing Practice
Logistics
Not only do we have logistics centers in eastern Japan (Kuki City, Saitama Prefecture) and in western Japan (Tosu City, Saga Prefecture) but we also have newly established Kansai Logistics Center (Kumiyama Town, Kuze County, Kyoto Prefecture) in April 2024, which underpin our logistics structure to maintain a stable supply of our products.
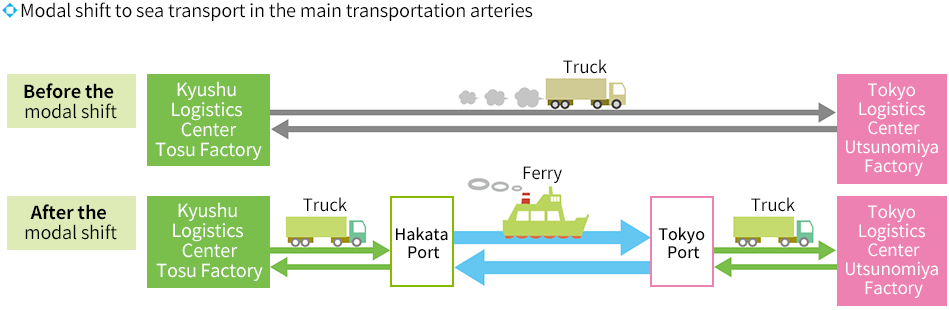
Staffed by supervising pharmacists to manage the quality of our products, each center shares information on its shipment volume together with relevant factories. This enables a wide variety of pharmaceutical products to be stored and managed properly and to be supplied to customers on a stable basis. Although many of our products are transported by land, we are making a modal shift in the main transportation arteries for the shipping of large-volume items from road transport (trucks) to sea transport, which has less impact on the environment. While expanding this modal shift on a continual basis, we will increase our loading efficiency and thereby strive to raise our transportation efficiency.
Modal Shift to Rolling Stock and Sea Transportation as the Main Transportation Channel
Sales/Marketing/Service
At sales/marketing/service, we provide and collect information on the effectiveness and safety of our products to enable medical workers and patients to use them with peace of mind and also to ensure appropriate use of the products. Additionally, requests from healthcare professionals collected by MRs are shared with related departments to further improve products, and the OTC business also promotes “Jissen” in which samples are distributed to directly communicate the superiority of products to consumers.
We help not only patients in the medical field, but also other people around the world maintain and improve their good health.
Japan - Rx business
Sharing of Information about Primary Palmar Hyperhidrosis
Not a few patients with primary palmar hyperhidrosis have given up on it as they thought of it as a matter of constitutional condition.
It is, therefore, important to deliver information to a wide range of patients and healthcare professionals so that they can understand that the disease is curable and improve the patients’ QOL. For patients, we are conducting raise awareness activities for disease through TV commercials, Web commercials, and online public lectures for citizens.
For healthcare professionals, on the other hand, we establish an education system for MRs to provide appropriate information and conduct workshops that allow healthcare professionals to simulate the patients’ symptoms of primary palmar hyperhidrosis in order to promote awareness and understanding of this disease.


Announcement

on the Website


Lectures
Japan - OTC Business
Measures Targeting Ethical Consumers and New Young Adults
In recent years, citizens have been increasingly conscious about ethical aspects such as environmental issues and human rights in their consumption behavior.
We think it important not only to take some measures, such as using media and SNS to disseminate the importance of ethical consumption and specific initiatives for it, but also to promote the visualization of the effect as products to appeal appropriately through packaging and sales situations.
In order to communicate the advantage of our products directly to customers, we distribute samples at events and others.




Information delivery through various channels
In order to deliver appropriate information to the users of our products and make them feel the effects more clearly, we are delivering various kinds of information.

Creating 90th Anniversary Business Cards made from the cloth used for SalonpasⓇ
SalonpasⓇ, which was launched in 1934, has marked the 90th Anniversary of launch in 2024. This is all thanks to our many customers who have used SalonpasⓇ for a long time.
The creation of business cards realizes the idea that a certain group came up with when working on the theme “Proposals for new initiatives in eco-friendliness” in the in-house training session.
In the process of manufacturing SalonpasⓇ, it is inevitable that the loss of cloth occurs. Such cloth had been normally reused in the waste-to-energy process. In the group discussion to seek for any other method of using it during the training session, the technique for reusing such cloth as business cards was found. So, as one of measures for expressing our gratitude for commemorating the 90th Anniversary of SalonpasⓇ to customers and one of projects that would lead to our commitment to sustainability and improvement in employees’ awareness of eco-friendliness, we created business cards made from cloth used for SalonpasⓇ to commemorate its 90th Anniversary.

for Salonpas AeⓇ.
Overseas - Rx and OTC Business
May 18 is the SalonpasⓇ Day
When SalonpasⓇ was first launched (90 years ago), activities of sampling at public bathhouses for enhancing recognition and experience of use were started in order to let customers know how good the product was. No matter how we explain the product, it would be impossible to deliver all its features. One look is worth a thousand words. If you use and experience it, you can understand how good it is. In our company, sampling activities are at the center of our sales activities, called “Jissen” (literally, practical advertising). And in order to recognize it again, we conduct sampling activities on May 18 on a global basis.
Globally, there are many countries with no culture of transdermal patches. Now matter how hard we try to explain that the bestselling transdermal patch in the world is SalonpasⓇ, it may be hard to imagine it in countries with no culture of transdermal patches. Therefore, we think that sampling for experience is an opportunity to judge the quality of the product, and therefore we implement sampling through events other than the SalonpasⓇ Day as well.
In the case of sampling in countries we newly launch the product, at first people sometimes say with a questioning look, “What is this?” But as the product permeates, we come to hear people saying, “I have used it,” and after that, we often come to receive a word of “thank you.” Stimulated by receiving a word of thanks more frequently, we are thankful and encouraged by the fact that our activities are making our customers happy while conducting our sales activities.



Implementation of Various Activities for Overseas Expansion
We are promoting a wide range of activities, including information delivery using various SNS services, participation in community events, building partnerships with clients in each country, and personnel exchanges within the Group.



Halal* Certification
Hisamitsu Pharmaceutical operates businesses in Indonesia, Malaysia, and other countries and regions where many Muslims live.In our subsidiary Hisamitsu Indonesia, in order to ensure that the haral guidelines in Indonesia are followed, and Muslims can use our products with peace of mind, we have established a Halal team within the Company and are promoting the creation of products that consider religion and the lifestyle of region.*Halal: What is legal under Islamic law

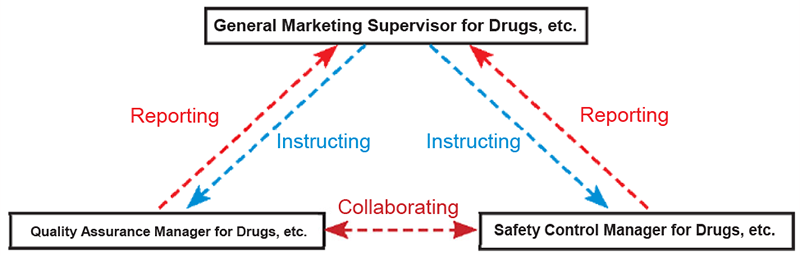
Quality Assurance
Quality is assured through GMP-compliant and scientifically established methods of production to ensure the reliability and safety of drugs.
Our quality assurance operations range extensively from “preparing process charts related to production and quality,” to “inspecting, testing and evaluating products,” “analyzing defect trends,” “auditing and instructing business partners,” and “correspondence with customers after shipment.” Furthermore, we carry out a wide range of internal and external quality assurance, including the creation of a smooth transition from the formulation development stage to the production stage to ensure quality assurance structure and management and auditing of the production system of contractors that manufacture OEM (*) products.
We have established an internal compliance promotion organization to monitor compliance with strict laws and ordinances and voluntary regulations. Additionally, we are constantly striving to improve quality and safety of our drugs under the strict quality control system, including inspections of our quality system by public agencies.
*OEM: Original Equipment Manufacturer
Commitment to Product Reliability Assurance
Since pharmaceuticals are life-related products, strict management standards are established to ensure their reliability, including GLP*1 and GCP*2 at the research and development stage, GMP*3 at the manufacturing stage, and GPSP*4 and GVP*5 at the post-marketing stage. Our Quality Assurance & Pharmacovigilance Division conducts rigorous audits focusing on control standards at each stage of product development, ultimately ensuring product reliability through a system of reliability assurance covering the entire product lifecycle. Furthermore, in line with the globalization of the Company, we are working to strengthen our auditing system by expanding the scope of risk assessment as well as complying with the latest regulations and guidelines in each country.
*1 GLP:Good Laboratory Practice Standards for practice of nonclinical studies for the safety of drugs.
*2 GCP:Good Clinical Practice Standards for practice of clinical studies of drugs.
*3 GMP:Good Manufacturing Practice Standards for production management and quality control of drugs and quasi-drugs.
*4 GPSP:Good Post-marketing Study Practice Standards for post-marketing research and practice of study on drugs.
*5 GVP:Good Vigilance Practice Standards for post-marketing safety management of drugs, quasi-drugs, cosmetics, medical devices and regenerative medicine.
Supplier Audits
In FY2023, we conducted written, online, and on-site audits of more than 40 raw material suppliers and product manufacturing contractors in Japan and abroad to confirm that manufacturing and quality control of raw materials and contracted products were being performed properly. The audit items are set mainly to cover important matters related to the operations of drug substance, raw material, and contracted product manufacturing plants; testing facilities; and other facilities based on GMP and other standards. Additionally, in the event of quality-related changes or complaints, we conduct a special audit to ensure product quality and stable supply.
Inquiries from Customers
At Hisamitsu Pharmaceutical, we have the Customer Center as a contact point for customers to make inquiries about our products. We respond to not only inquiries through our website, but also inquiries from individual customers by phone and letter.
While striving to make attentive, prompt, and appropriate responses to such inquiries, we share feedback and indications from customers internally among related departments in order to help improve the relevant products or develop new products.
